Solutions Built for Life Sciences Manufacturing
Life sciences manufacturers are dedicated to innovation, product quality and patient safety, but a tightening regulatory environment, increasing cost pressures and supply chain complexities are causing new challenges. Emerging global markets, quality initiatives, and increasing mergers and acquisitions activity add to the complexity of life sciences manufacturing and distribution.
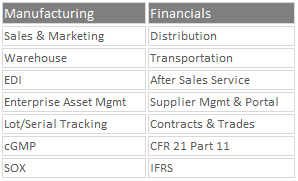
QAD Cloud ERP enables medical device, biopharmaceutical, and contract manufacturers to effectively:
-
- Facilitate innovation and drive greater operational efficiency
- Conserve capital and support acquisitions and divestitures
- Integrate quality functions into core business processes
- Manage supply chain planning and optimize distribution
- Reduce the risk of non-compliance
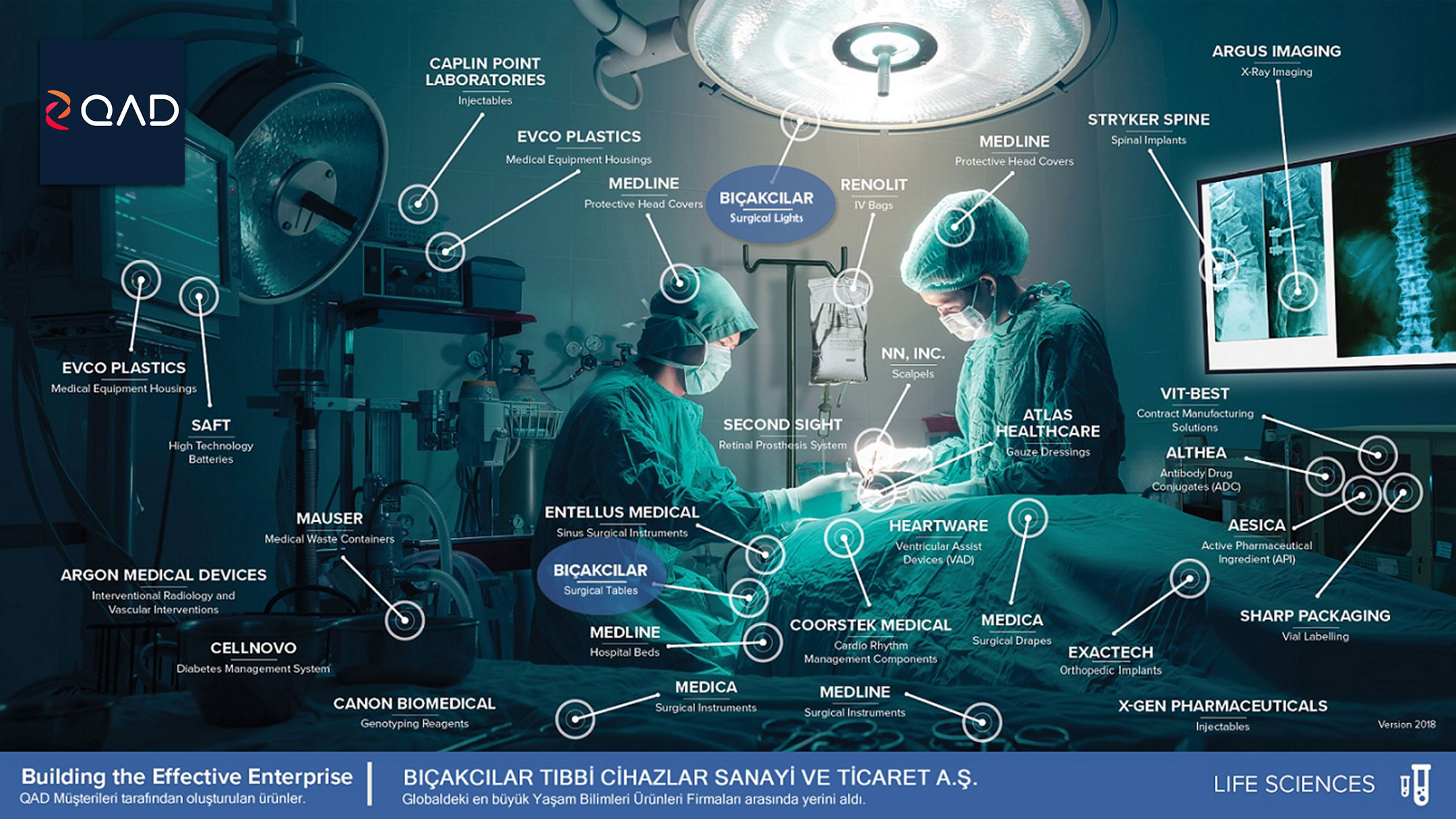
Over 400 life sciences manufacturing sites in more than 40 countries around the world have deployed QAD Enterprise Applications. Companies ranging from start-ups to multinationals enjoy the simplicity, reliability and performance that QAD delivers.
QAD developed the Life Sciences Edition to meet the needs of its worldwide customers in a variety of segments from medical devices to pharmaceuticals. QAD’s commitment to life sciences ensures medical device, pharmaceutical, and biotechnology manufacturers are able to work towards becoming effective enterprises, while meeting stringent regulatory demands.
QAD‘s solution supports flexible business process configuration and multiple business entities and can be deployed in multiple languages. Deployment options — including on demand — support the needs of global life science companies.
QAD’s solution supports critical quality requirements, including regulatory compliance, CAPA, and Device History/Electronic Batch Record requirements as well as providing a full range of Current Good Manufacturing Practices (cGMP) validation capabilities and tools. Enhanced controls and audit capabilities, along with eSignature support, help meet the mandate of compliance tracking.
QAD Lets You Work Faster, Work Smarter:
- Full IQ/OQ/PQ Validation Support
- Integrated Device & Batch History Report (DHR)
- Robust Cradle to Grave Lot Traceability
- Intuitive and Easy to Use Interface
- Qualified SaaS Environment
- Built in Industry Best Practices & Processes
- 21 CFR part 11 Compliant
- FDA Regulatory Compliance
Web-based ERP Software – for Life Sciences
QAD is committed to helping manufacturers in the Life Sciences Industry. Another way QAD has made organizations more effective is through our web-based ERP Software – QAD On Demand. This web-based ERP solution allows Life Sciences companies to focus on their business, not their software. The online functionality of our ERP software greatly reduces the cost of implementation and need for IT maintenance.
QAD On Demand Life Sciences Edition offers the same capabilities as the On Demand General Edition, but provides a qualified environment and the necessary standard operating procedures (SOPs) required to gain Food & Drug Administration (FDA) and current Good Manufacturing Practices (cGMP) validation.
QAD recognizes the trust that our customers place in us as their Enterprise Resource Planning solution provider. This is why so many Life Sciences companies have chosen QAD.
Life Sciences On Demand
Clear Benefits
- Hosted ERP Solution
- Reach Benefits Faster
- Lower Cost Structure
- Standardized Process Maps
- cGMP & CFR 21 Part 11
- Global Support
- Quick Implementation
- No Capital Investment
- Best Practice Configuration
- SOX & IFRS
- FDA Compliant Services
Value Added – Hosting Services
- Qualified Hardware
- Qualified ERP Installation
- FDA Compliant
- Dedicated Single Tenant
- Qualified Operating System
- 18 Dedicated SOPs
- Additional Validation DB
- Flexible Upgrade Time
ERP Focused on the Life Sciences Industry
From its inception QAD has focused on the manufacturing sector with a strong emphasis on the Life Sciences industry. This unwavering commitment has enabled us to bring the best ERP solution to help drive the success of our customers.
On Demand ERP Benefits
SME, M&A, Growth, and Multi-Nationals
Start-ups & medium size enterprises, companies with mergers & acquisition activities, or companies expanding globally by adding new sites in new countries are attracted to QAD’s On Demand ERP offering for scalability, reliability and risk mitigation.
QAD offers the perfect hosted ERP solution that meets your unique global business and manufacturing needs, therefore enable you to meet your company objectives and regulatory compliance.Easy-On-Boarding Implementation
Configured with built-in industry best practice processes, implemented with wizard style Easy-On-Boarding methodology, QAD On Demand delivers full suite ERP & Supply Chain business solution with the best industry fit and the record implementation time. This agility allows our customers to reap the benefit of their investment in technology quickly.
Flexibility and Scalability for the Life Sciences Industry
Driven by more demanding regulators and customers, today’s Life Sciences industry has to adapt to ever increasing regulatory and market changes. QAD’s On Demand provides the perfect platform for quickly aligning your business to these changes, therefore allow you to focus on your business, not your software.
Business Value for Life Sciences Companies
QAD On Demand delivers not only out-of-the-box cGMP and CFR 21 Part 11 compliance, but also financial compliance with SOX, GAAP, and IFRS. 24×7 services, 99%+ up time, strictly followed SOPs, and security rich features make QAD the safe and secure ERP choice of any Life Sciences companies.
QAD On Demand service organization is your extended IT arm. Operated under strict FDA CFR 21 Part 11 requirements with 17 dedicated SOPs, we qualify the hardware, operating system and computer system installation for you, mitigating your compliance risk and providing best in class IT operational control.